New Zealand Doctors Speaking Out with Science
Maria Gutschi is a Doctor of Pharmacology with vast experience in analysing good manufacturing practices. She recently presented an ‘Independent Analysis of Quality Issues of the BNT162b2 BioNTech/Pfizer Vaccine Identified by the European Medicines Agency‘.
The 40 minute presentation was delivered to Team Enigma, a group of scientists investigating the inconsistencies in Covid-19 vaccine products which strongly suggest that good manufacturing practices are disregarded at multiple levels, from raw materials to every step in the production process. Team Enigma’s website How Bad is My Batch reports close to 34 million visitors to date. Their evidence is consistent with concerns being raised persistently by NZDSOS scientists alongside international teams.
Our appeals for investigation are repeatedly ignored by New Zealand authorities.
Good Manufacturing Practices : Concerns With Pfizer’s Comirnaty
Dr Gutschi’s presentation is quite technical, so we have attempted to summarise the main points here. A most useful reference she provides is How to Make Enough Vaccine for the World in One Year.
The European Medicines Agency (EMA) are Dr Gutschi’s preferred reference for drug assessment work. She describes them as being more complete, organised and transparent than their North American counterparts, the US Food and Drug Administration and Health Canada. Data is usually reviewed once trials are completed and the data is collated, but BNT162b2 (Comirnaty) was reviewed as data became available, in what Dr Gutschi calls a ‘rolling review’ which was more difficult. The pivotal trial data was published in the New England Journal of Medicine on 10 December 2020 and EMA approved Comirnaty under Conditional Marketing Authority (the European equivalent of American Emergency Use Authorisation) on 20 December 2020.
Dr Gutschi describes Comirnaty as the most complicated product she has ever seen, involving 280 components, multiple manufacturing plants and suppliers, ultra-cold storage requirements and rapid transport between sites, often on different continents. The complicated process impacted access to other products as production lines were disrupted during the rush to supply Comirnaty. In April 2021 Dr Gutschi discovered leaked confidential documents, filling in gaps to her unanswered questions during review of the EMA data.
She provides a detailed description of mRNA production, which EMA defined as the active drug substance. She emphasises that these are synthetic and genetically engineered substances, and neither human nor biological. Her slides explain the use of pseudouridine in the mRNA as a replacement for uridine, as a way of increasing the amount of protein synthesised. This has also been described by pathologist Dr Ryan Cole in this brief clip from his full 30 minute interview with Roman Balmakov in April 2022.
Consistent with Dr Cole’s concerns, Dr Gutschi describes how this genetically engineered product can lead to multiple health issues including auto-immunity, immune suppression, cancer and neurotoxicity risks.
Concerns about the mRNA then lead into concerns about the spike protein that the injected mRNA produces within the body. Initially requiring that Pfizer provide more detail about the spike protein, which should have been a simple process, when this requirement was not met, the EMA brushed this requirement aside. This is a clear breach of good manufacturing practices.
Dr Gutschi explains the difficulties involved for BioNTech, who had never brought a product to market before, to upscale their vaccine production from a few thousand to many millions of doses. She then shows how this breaches good manufacturing processes and compromises product purity and integrity. Her review of the EMA notes shows that they experienced a “tonne of consternation” over this issue. When they asked Pfizer to provide details such as how to ensure truncated and fragmented mRNA, which could translate into proteins leading to auto-immunity, would not remain in the final product, answers were never forthcoming.
Good manufacturing practices were ignored and the regulator allowed it.
She then provides details about the lipid nanoparticles (LNPs), some of which had never been used in humans before. In 2020 there were only 200 people in the world who knew how to make LNPs, requiring mass recruitment and training of many more people in use of the specialised machines involved. Dr Gutschi provides illustrative evidence that the final LNPs do not meet usual standards for good manufacturing practices, raising multiple concerns.
Multiple other concerns are outlined including ways to determine potency of the product, ways to identify the clinical impact of the product, and variability between batches of product. She describes her horror that shards of stainless steel, visible to the naked eye, were found in a Moderna product in Japan, and the unknown connection between this and three deaths.
In normal circumstances such violations would result in the closure of the manufacturing facility.
Many errors have occurred including administration, dosing, reconstitution and others. Dr Gutschi illustrates the poor systems which have likely contributed to this, such as similarities between labels of different products and errors on labels attached to products which were distributed. She describes these issues as ‘unconscionable’.
Her final two slides are of particular interest. Her analysis summary:

Dr Gutschi is now researching the “pharmacokinetics” of these products. This means where they go in the body, where and how they are absorbed, how the body processes them and how they are eliminated from the body. She is working to understand the sources of variability in individual responses to the product, and finishes the presentation with her current findings:
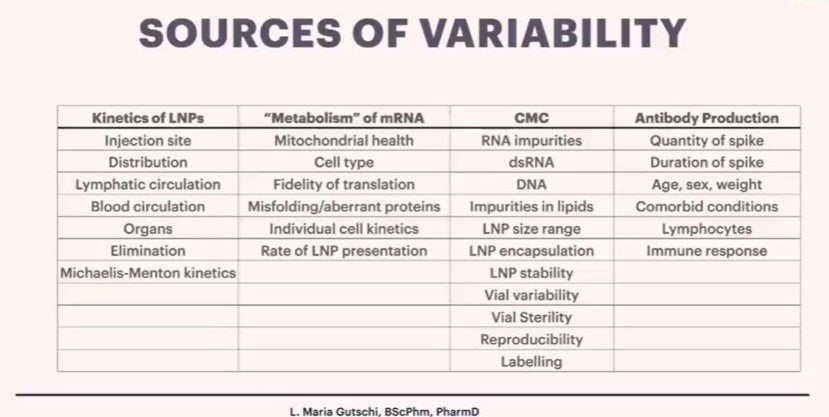
In ordinary times, this product would be immediately pulled from the shelves, those involved would be named by the mainstream media, investigations would be held by regulatory authorities as to how this was allowed and compensation provided to those affected.
New Zealand was told that the Comirnaty injections would be independently analysed. Not only has this not happened but the regulator, Medsafe, has turned a wilful blind eye to obvious problems with manufacturing. NZDSOS continues to ask what influence Pfizer has, that has led to these blatantly obvious manufacturing problems being ignored?
Watch: Independent Analysis of Pfizer’s Good Manufacturing Practices
The full 40-minute presentation is available on the Team Enigma Bitchute channel, or watch it here.

