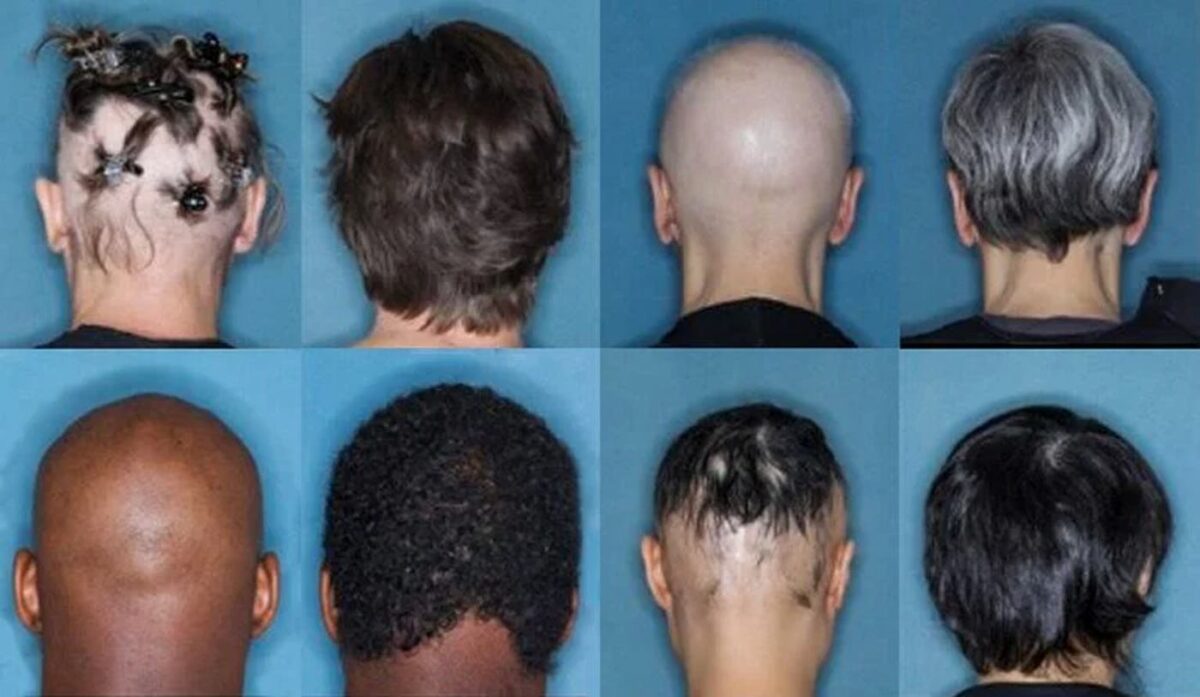
In the trials, Olumiant helped one in three patients with severe alopecia areata regrow their hair—almost half of the patients had no scalp hair at the start of the trials—resulting in 80% or more scalp coverage. Improvements were also achieved for patients with significant eyebrow or eyelash hair loss.
[…] “Until now, there have been no FDA-approved treatments for alopecia areata,” King explained […] FDA approval will bring greater access, via insurance coverage, to patients.”
[…] He remembers the first patient he treated. “He had almost no scalp hair, his eyebrows and eyelashes and facial hair were missing, and, in addition, he had red, scaly psoriasis plaques all over his body. It was in 2013… I explained to the patient that use of tofacitinib in him would be exploratory, and he agreed to try it…. Not long after he started taking tofacitinib, his hair started to grow. I published the results of his treatment not long after that and history was made, forever changing this disease.”
goodnewsnetwork.org/fda-approves-first-systemic-treatment-alopecia-olumiant/
Good News of the Day
FDA approves first Alopecia drug that restores hair growth in many patients